Electrical Hyperexcitability in Alzheimer’s: The Emerging Seizure Link
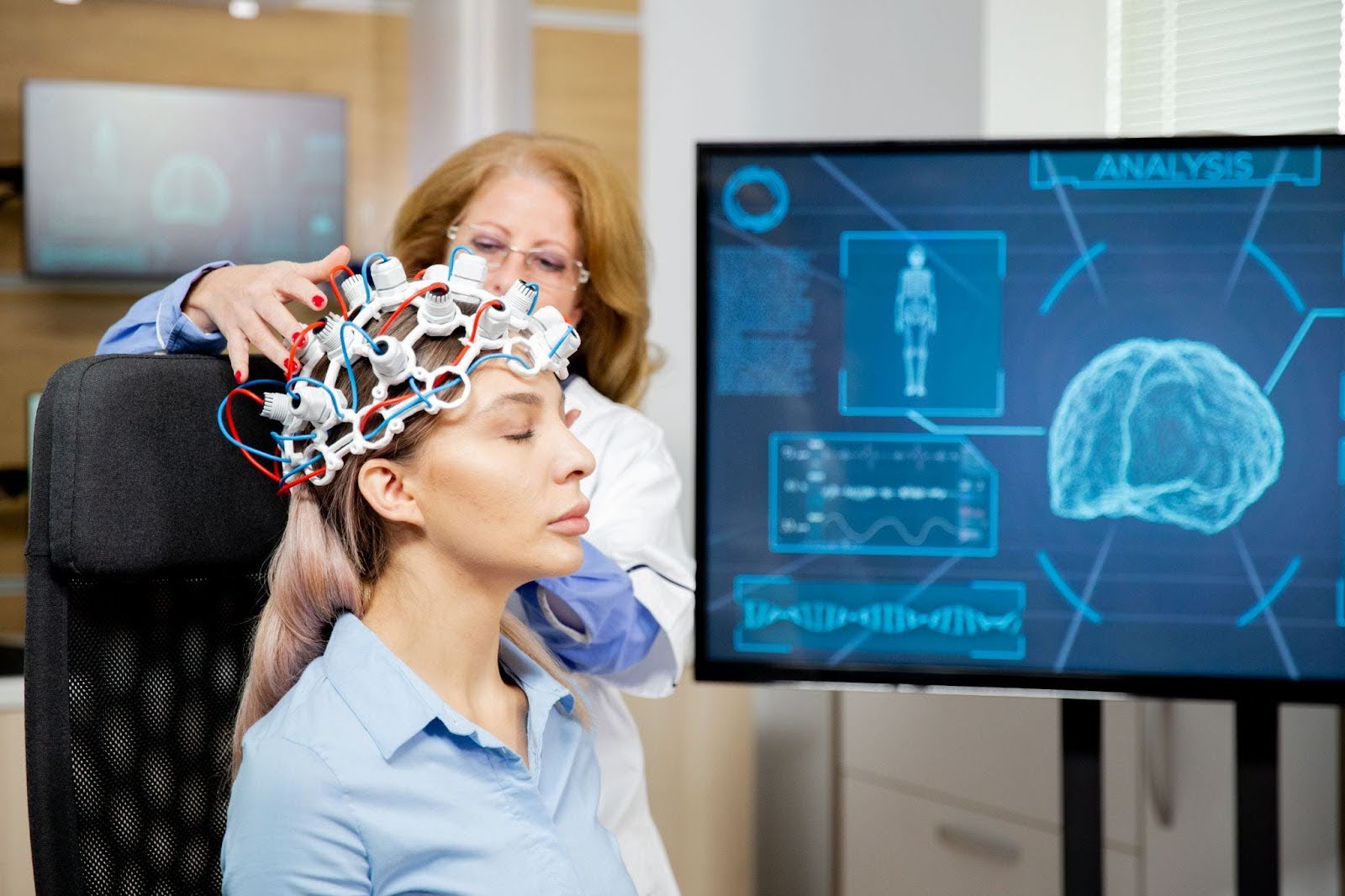
Alzheimer’s disease has long been framed through the lens of memory loss, protein plaques, and cognitive deterioration, but a growing body of research is uncovering another powerful dimension of this condition: electrical instability within the brain. A Neurologist examining early-stage patients is now more likely to observe subtle patterns of abnormal neuronal firing that precede visible symptoms. Long before noticeable cognitive changes emerge, neurons in vulnerable regions especially the hippocampus and temporal lobes begin firing at destabilized rates. This phenomenon, known as electrical hyperexcitability, is reshaping how clinicians and researchers view the early trajectory of Alzheimer’s.
For decades, Alzheimer’s and epilepsy were treated as unrelated neurological landscapes, one dominated by memory failures and cognitive erosion, the other by convulsive episodes and overt electrical discharges. That separation is rapidly dissolving. Across multiple clinical studies, patients in the early stages of Alzheimer’s show a significantly higher incidence of seizures, even when no visible convulsions are present. Many of these episodes go unnoticed by patients and caregivers, occurring silently in the background, eroding cognitive stability and accelerating decline.
A key driver of this disruption lies in the collapse of electrical balance across neural circuits. The healthy brain maintains a delicate ratio between excitatory and inhibitory signaling. When GABAergic interneurons which provide braking power against excessive firing are damaged or reduced, the excitatory glutamate pathways dominate. Alzheimer’s pathology interferes with these systems on multiple levels. Amyloid-beta plaques cling to synapses and alter neurotransmitter behavior. Tau tangles impair microtubule stability, disturbing neural transport and function. The resulting imbalance forces neurons into hyperactive states that set the stage for epileptiform activity.
One of the most striking revelations of recent research is that these seizures are often not the dramatic kind people expect. Instead, they manifest as microscopic electrical surges known as subclinical or silent seizures. These events can occur during sleep, periods of rest, or even while a person appears alert. They may last only seconds, but the cumulative damage they inflict on memory consolidation and network communication is profound. Individuals may experience short lapses in awareness, abrupt confusion, or sudden disruptions in speech all easily mistaken for general cognitive symptoms of dementia rather than seizure-related events.
The hippocampus stands at the epicenter of this electrical vulnerability. As one of the first regions attacked by Alzheimer’s pathology, it becomes both a victim and a catalyst of hyperexcitability. Researchers have discovered that neuronal networks within the hippocampus begin displaying excessive firing patterns even during the earliest cognitive shifts associated with mild cognitive impairment. This activity does not always represent dysfunction in its initial phase. Some experts theorize that hyperexcitability may begin as an overcompensation mechanism, as compromised neurons attempt to maintain information transfer. Over time, however, the constant, unregulated firing exhausts the circuit and accelerates neural death.
Sleep further complicates this relationship. Deep sleep is vital for clearing metabolic byproducts and stabilizing memory traces. In brains affected by Alzheimer’s, restorative sleep is often disrupted by bursts of hyperactive electrical signals. These interruptions weaken glymphatic clearance processes, disturb synaptic recalibration, and prevent the neural recalibration needed for cognitive resilience. What was once viewed simply as insomnia or restlessness in older adults is now being reconsidered as a symptom of seizure-linked dysfunction.
Diagnostically, the silent nature of these events presents a critical challenge. Standard EEG recordings often fail to capture the brief and sporadic discharges occurring deep within temporal structures. As awareness grows, more clinicians are considering long-term EEG monitoring—particularly during sleep—for individuals experiencing rapid cognitive decline, early-onset symptoms, or unexplained behavioral fluctuations. Early identification could transform therapeutic strategies before irreversible damage sets in.
Treatment approaches are now beginning to adapt to this evolving understanding. Certain antiepileptic medications are being evaluated not only for seizure suppression but also for their potential neuroprotective effects. Drugs such as levetiracetam have demonstrated the ability to reduce hippocampal hyperactivity and, in some cases, improve memory performance in early-phase patients. These findings highlight an emerging consensus: electrical stabilization may play a pivotal role in slowing the cognitive descent linked to Alzheimer’s.
The broader implications of these discoveries are reshaping models of neurological care. Rather than viewing Alzheimer’s as a static, degenerative condition, researchers are increasingly characterizing it as a dynamic disorder influenced by electrical, biochemical, and immunological disruptions. Precision-based neurology which incorporates electrophysiological screening, molecular profiling, and early intervention could eventually become the standard rather than the exception.
Another dimension gaining traction involves how neurological damage interacts with psychiatric and behavioral conditions. Brain regions affected by dementia often overlap with those impacted by stress, addiction, and trauma. Substance-linked alterations in neural chemistry, particularly involving inhibitory and excitatory signaling, may intensify susceptibility to hyperexcitability. As seen in discussions on co-occurring neurological and mental health vulnerabilities, the relationship between cognitive dysfunction and external stressors adds complexity to diagnosis and management. For those interested in how these interactions shape broader brain health, this resource offers meaningful perspective: https://www-wellhealthorganic.com/alcohol-addiction-and-mental-health-understanding-dual-diagnosis/ Ultimately, the recognition of electrical hyperexcitability as a core feature of Alzheimer’s is reshaping the future of treatment and research. Rather than being interpreted as a downstream symptom, seizure activity is now considered a potential driver of disease progression. By detecting and targeting these electrical shifts early, clinicians may be able to preserve cognitive function longer, delay structural damage, and offer patients a more proactive path forward. The fusion of neurophysiology, cognitive science, and precision medicine is opening a frontier where the brain’s electrical language may reveal the earliest signs of decline—and the most promising avenues for intervention.
Keep an eye for more latest news & updates on Wellhealth Organic!